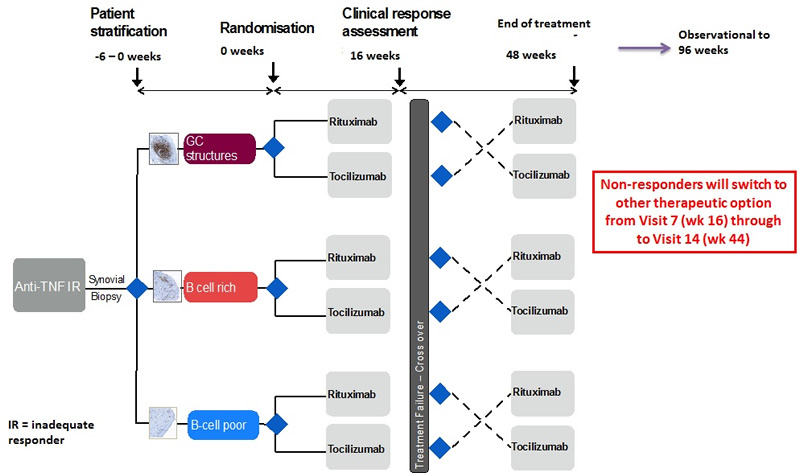
Patient Flow Diagram
The main aim of this project is to test the hypothesis that the presence or absence of specific synovial cellular and molecular signatures (B cells and B cell-associated signatures), assessed following a synovial tissue biopsy, will enrich for response/non-response to the B cell depleting anti-CD20 monoclonal antibody (mAb) Rituximab. In addition, we will examine if clinical response is associated with inhibition of B cell-linked pathways within the synovium and dependent on local B cell lineage depletion and whether survival of auto-reactive B cells within "protected" synovial niches are responsible for B-cell joint re-population and disease resistance-relapse?
The overarching hypothesis is whether a diagnostic synovial biopsy showing a "B-cell rich/poor pathotype" can define specific disease responsive/resistant subsets for patient stratification and help rationalize biologic drug choice.
Therefore, while this study can be thought of as taking place in three distinct and separate synovial histomorphological phenotypes (B cell rich, B cell poor and Germinal centers):
- The primary aim of this project is to show that in patients failing anti-TNF therapy, with a B cell poor synovial pathotype, Rituximab is inferior to Tocilizumab therapy.
- For the B-cell rich synovial pathotypes, we aim to show non-inferiority of Rituxumab compared to Tocilizumab.
- Germinal Centre pathotypes will constitute an exploratory component to the trial as insufficient power will be generated to show a significant difference in clinical response between each treatment.
This is a open labelled, randomised clinical trial. Patients recruited to this study will undergo a synovial biopsy at baseline, prior to randomisation. Patients will subsequently be stratified in to 3 groups (B Cell Poor, B Cell Rich, Germinal Centres (GC) Rich) according to the following B-cell score prior to therapeutic intervention.
The study is led by the Centre for Experimental Medicine and Rheumatology at Barts and the London School of Medicine and Barts Health NHS Trust.
There will be approximately 24 additional centres participating in the trial. The study commenced in February 2013 and we plan to recruit 180 patients over a 24 month period. Patient follow-up is a total of 96 weeks duration, consisting of 48 weeks treatment phase + 48 weeks observational phase.
This study is being funded by the National Institute of Health Research Efficacy and Mechanism Evaluation program (NIHR EME)