Aims & Objectives
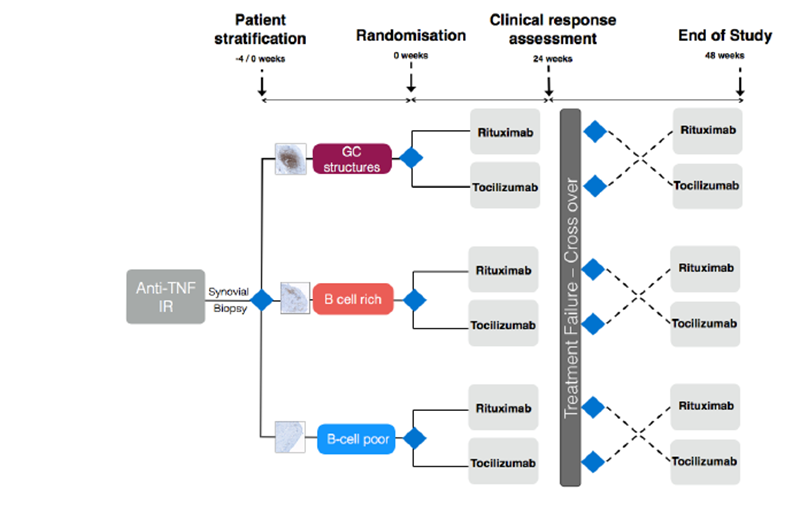
The overall aim of this study is to develop a novel, biopsy-based pathway in which patients are stratified to treatment based on their synovial pathotype.
Our primary aim is therefore to test the hypothesis that patients with RA who are inadequate responders to anti-TNF therapy and have a “B-cell poor“ synovial pathotype will exhibit an inferior response to Rituximab when compared with Tocilizumab than those with “B cell rich“ pathotypes.
Following the same biological principle, we aim to show that in those with “B cell rich” synovium, rituximab is not inferior to tocilizumab. This will be addressed by randomisation to either RTX or TCZ, with inadequate responders switching to reciprocal therapy at six months. This may have strong implications on current NICE guidance if tociluzimab is shown to be superior in those not responding to anti-TNF therapy regardless of synovial B-cell pattern.
At a molecular level we aim to elucidate mechanisms involved in response/non-response to B-cell depletion. We will endeavour to ascertain whether clinical response is associated with inhibition of B cell-linked pathways within the synovium and dependent on local B-cell lineage depletion and the presence of germinal centres. Furthermore we will investigate whether disease relapse, which usually occurs 6-9 months after treatment with Rituximab, is related to survival of auto-reactive B-cells within “protected” synovial niches. Pre- and post-treatment synovial biopsies will be examined using high-throughput technologies and pathway analysis which will allow the search for novel biomarkers and predictors of clinical response and further our understanding of the molecular mechanisms involved in response and resistance.